Herbicide resistance has been called the number one threat to conventional herbicide-based weed management strategies.
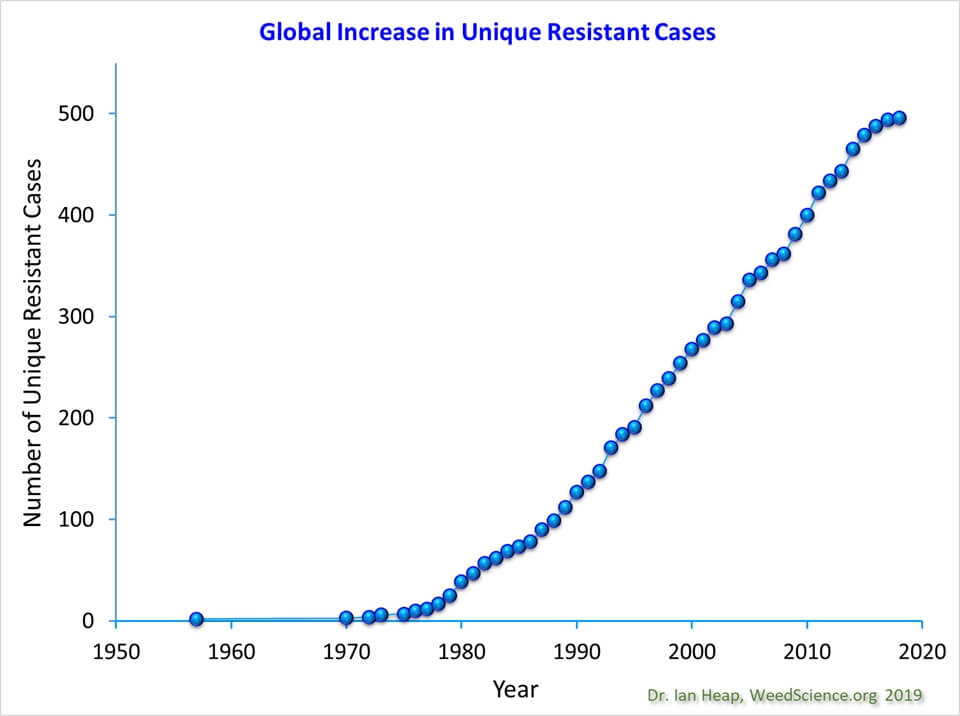
Since the 1970s, the number of cases of herbicide resistant weeds has shown a linear increase both globally (currently at about 500 documented unique weed species x mode of action cases) and within Canada (at about 70 such cases), according to the herbicide resistance website WeedScience.org. The rate of increase has been constant, and there is not yet any reason to believe that growth in the number of cases will slow.
By using herbicides, we select for weed biotypes that, for some reason, can tolerate the product. Mutations which confer herbicide resistance are rare, but present at very low levels in most weed populations. Repeated use of the same mode of action will increase the relative frequency of the resistant biotype until it becomes noticeable, and shortly thereafter, problematic.
The best-known forms of resistance involve single-gene mutations that alter herbicide target sites (target sites might be enzymes that produce essential plant cell building blocks) so that herbicide binding is reduced, resulting in reduced control. As a result, the target pathway keeps working, and the plant grows normally after herbicide application. Other forms of resistance involve the overproduction of the target enzyme by the plant, mechanisms that either metabolize or sequester the herbicides, or changes in uptake of the herbicide. The main mechanisms are summarized in this table:
Table 1: Mechanisms of herbicide resistance*
| Resistant Class | Mechanism |
|---|---|
| Target Site | Target site mutation |
| Increased gene copy number | |
| Enzyme over-expression | |
| Non Target-Site | Enhanced metabolism |
| Differential uptake | |
| Differential redistribution | |
| Sequestration | |
| Delayed germination | |
| Rapid necrosis / defoliation |
*Source: Bo AB, Won OJ, Sin HT, Lee JJ, Park KW. 2017. Mechanisms of herbicide resistance in weeds. Korean Journal of Agricultural Science 44:001-015.
The simple act of using a herbicide can select for resistance to that herbicide. While we can’t predict or prevent resistance entirely, we can slow its onset by reducing the frequency of herbicide use, for example by integrating cultural controls such as crop rotation, seeding rate, cultivar competitiveness, and other factors into our agricultural systems.
A powerful option to slow resistance development is to reduce our reliance on a single mode of action, either by rotating modes of action in successive sprays, or, more importantly, by tank mixing multiple effective modes of action (MEMoA) whenever we make an application.
Let’s not kid ourselves. The recent discovery of glyphosate (e.g. Roundup) -resistant wild oats in Australia, and glufosinate (e.g. Liberty) -resistant ryegrasses in several countries is sobering. Relying more on these herbicides will only increase selection pressure.
If we decide to use herbicides, we need to look at the situation from the perspective of delaying the onset of resistance. What we’re trying to do is buy some time, so that new strategies can be developed.
How can spray application methods slow the onset of resistance?
The use of herbicides will continue to select for resistance. The best we can hope to achieve within a herbicide system is to delay that eventuality.
To better understand our options, we need to talk about a specific type of herbicide resistance called polygenic resistance. This refers to accumulation of additive genes of small effect over time, a process that is more efficient in plants that share genetic material among plants in a population, i.e., they outcross.
Outcrossing plants receive genetic material from others, increasing their genetic diversity, and therefore their ability to adapt.
In a field, a population of any specific weed may contain some individuals that have slightly greater tolerance to a herbicide than others. If we apply a slightly lower than label herbicide dose to those individuals, they might survive the application and eventually cross with other survivors and set seed. Their offspring may be as tolerant or even more tolerant than their parents. If this repeats itself over successive generations, the additive effects build until finally, low-level resistance becomes full-blown resistance and even label rate herbicides no longer work. This resistance isn’t a single gene mutation, it’s simply an accumulation of tolerance due to several genes which impact how much of the herbicide active ingredient reaches the target site.
In a recent study at the University of Arkansas, susceptible Palmer amaranth (P. amaranth has both male and female plants and is therefore an obligate outcrosser) was treated with a range of dicamba doses to identify individuals that survived the higher doses. The researchers allowed the survivors to cross, and then grew out their seed, then repeating the procedure. After just three generations, the experiment produced individuals with a three-fold increase in LD50 (compare LD50 at P0 (111) to P3 (309) in Table 2). Recall that LD50 refers to the dose required to observe 50% of the full effect.
Table 2: Dicamba doses (g ae/ha) required for 50% (LD50) and 90% (LD90) control of Palmer amaranth populations selected following sublethal doses of dicamba in the greenhouse.*
| Herbicide | Selected Population | LD50 | LD90 |
|---|---|---|---|
| Dicamba | P0 | 111 | 213 |
| P1 | 198 | 482 | |
| P2 | 221 | 546 | |
| P3 | 309 | 838 |
The lessons are three-fold:
- Herbicide resistance cannot be prevented if herbicides are applied.
- To prevent polygenic resistance, we need to apply the full label rate and avoid repeated sublethal doses, so that all weeds are killed;
- We need to apply Multiple Effective Modes of Action (MEMoA) whenever possible so that when one fails, the others have its back;
How can this be achieved?
- Prevent application practices that result in less effective dosing. Larger weeds, or weeds growing in difficult environmental conditions, may require higher herbicide doses. Early application is helpful because small weeds are easier to control. In addition, crop canopy shading at later staging leads to dose reduction and increases dose variability. Spraying under windy conditions also reduces dose, and can increase deposit variability. For some herbicides such as glyphosate or diquat, the dust generated by wind or fast travel speeds can reduce effectiveness.


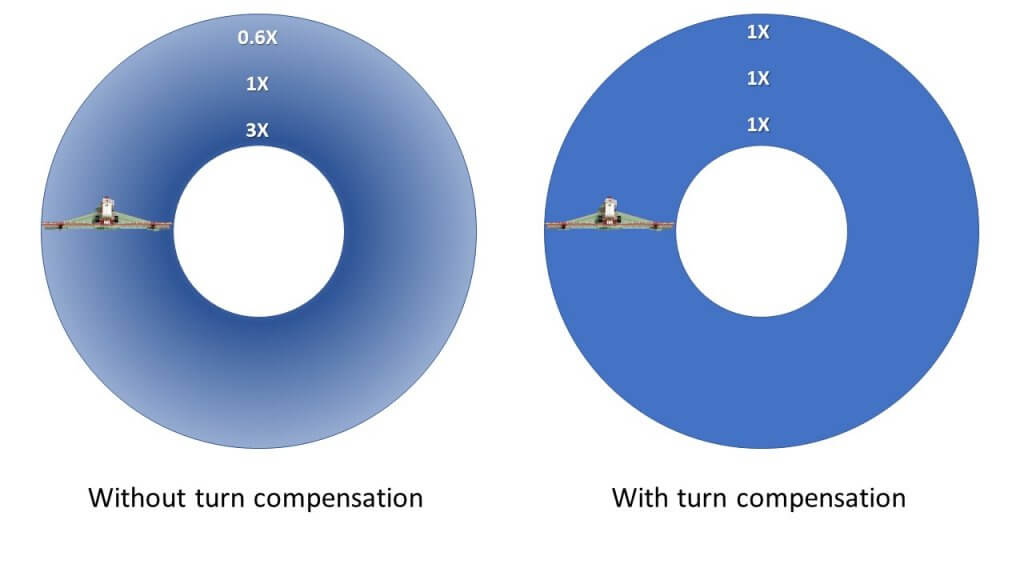
- Get Pulse Width Modulation (PWM) with turn compensation. If your sprayer makes the same turn around the same feature year after year, then the outer boom region will under-dose the same part of the field over and over. This is the breeding ground for polygenic resistance. Look for this in field corners, around water bodies or tree bluffs, rock piles, etc.
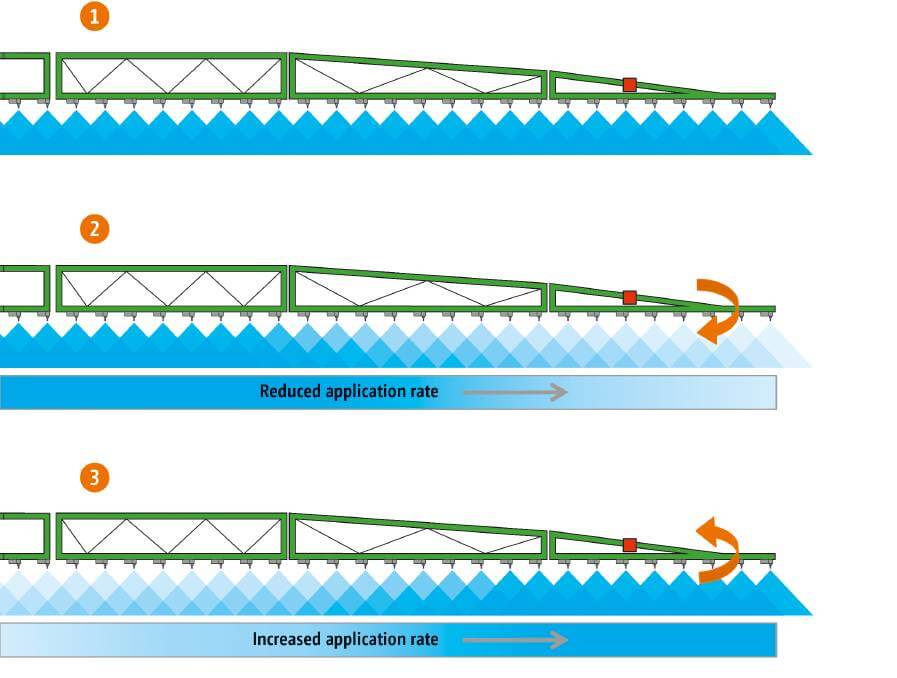
- Prevent boom sway and yaw. Boom movements result in uneven application, which results in lower control. Pull-type sprayers with supporting wheels are best, but these are becoming rare. Suspended boom performance depends on the manufacturer and the levelling technology they use. However, boom movement is usually more consistent with slower travel speeds.
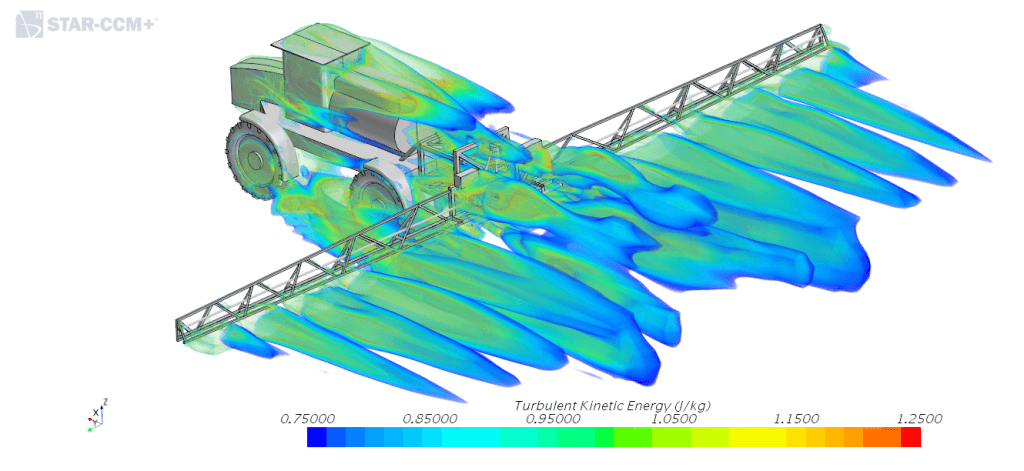
- Minimize air turbulence. Large sprayers, and those moving at fast speeds, create aerodynamic turbulence that can displace spray. The main problem spots are wheels, in whose tracks measurably less spray is deposited. The exact dynamics of turbulence is still unknown, but we do know that its magnitude can be reduced with slower travel speeds.
- Consider spot spraying. The use of optical spot spray equipment, such as the new WEEDit Quadro, or Trimble’s WeedSeeker II, save product during burnoff or post-harvest. These savings can make the use of more elaborate, expensive tank mixes containing multiple effective modes of action, affordable.

- Avoid spray drift. Field margins that harbour weeds rarely receive a full dose of herbicide. Exposing these weeds to spray drift won’t kill them. But it will, over time, select for weeds that are more able to tolerate the herbicide.
Implications
Aside from specific technology such as PWM, improved booms, or a spot sprayer, the most effective fix for variable application doses is slower travel speed.
While this may seem problematic when timing is critical and greater productivity is required, there is a way to drive more slowly and still get more done. We simply need to look at productivity differently.
We tend to equate productivity with speed. Travel speed. But a spray day is filled with many hours of non-spray time – filling, cleaning, transporting, repairing, fueling, record-keeping, etc. How much time is lost to these activities depends on the operation, but for everyone, it’s useful to do time accounting.
Record how a spray day’s time is spent. Pay attention to activities during which you can save time without much expense.
| Action | Actual Time | Target Time |
|---|---|---|
| Fuelling, lubing | ||
| Loading jugs and totes | ||
| Checking label (rates, rainfastness…) | ||
| Filling tender tanks | ||
| Loading sprayer (in yard) | ||
| Transport to field | ||
| Entering field data into monitor | ||
| Checking, recording weather | ||
| Checking for pest, stage | ||
| Changing nozzles | ||
| Spraying load | ||
| Unplugging / replacing nozzles | ||
| Replacing nozzle body | ||
| Making turn | ||
| Filling sprayer | ||
| Getting sprayer unstuck | ||
| Driving to tender truck | ||
| Waiting for tender truck | ||
| Spraying out tank remainder | ||
| Cleaning tank | ||
| Cleaning filters | ||
| Flushing boom ends | ||
| Loading sprayer (in field) | ||
| TOTAL |
On any given spray day, less time spent filling, or transporting, is credited to spray time. Our analysis shows that time lost to driving slower can more than be made up with these changes. The productivity gain gives more opportunity to spray under more ideal conditions that save yield and also ensure more uniform application.
Using productivity analysis, spraying can become more uniform and help delay the onset of resistance.
Note: The assistance of Dr. Charles Geddes, Research Scientist at AAFC Lethbridge, in drafting this article is appreciated.