I work in agricultural extension and I’m always on the lookout for new methods to help me achieve my goals. A big part of my job is to research and teach efficient, effective and safe crop protection practices, so it follows that I have to be able to evaluate the quality of a spray application. Fundamentally, there are two ways to do it:
- Wait to see if the pesticide did its job and protected the crop from weeds / bugs / disease.
- Don’t wait. Confirm your spray is depositing where you want it before committing to the application.
Three guesses which approach I advocate. So, how do you check spray coverage in a way that’s quick, cheap, easy and informative? Again, there are choices, but rather than simply list them I’ll add a little insight in the form of pros and cons.
| Method | Pros | Cons |
| Water sensitive paper | Relatively cheap, available, clean, easy, repeatable, supports a photographic record, simple to analyze. | Does not accurately reflect coverage on plant surface, slow to place and retrieve, can be spoiled by dew, humidity and physical contact. |
| Inspecting for residue / wetness | Cheap and fast. | Not proactive, too subjective, not repeatable, pesticide many not leave visible residue, requires re-entry soon after spraying. |
| Inspecting spray pattern (e.g. shoulder check) | Cheap and fast. | Not proactive, not indicative of coverage, not repeatable. |
| Watching for run-off | Just don’t. | Just don’t. |
| Fluorescent dyes | Reflects actual, whole-canopy coverage and off-target coverage at same time. | Expensive, hard to find, messy, time-consuming, hard to photograph, not repeatable, leaves unwanted residues (or can’t be used on edibles), may have to take place at night, may fade quickly… or is any of this actually true? |
I’ve never been a proponent of spraying dyes because of the reasons I listed in the table. If I already have difficulty convincing a grower to leave the sprayer or tractor cab to place and retrieve water sensitive papers, what are the odds of them mixing a messy and expensive tank of dye and waiting until twilight to see the results?
On the other hand, dyes are compelling. Particularly if we change the perspective a little. What if we consider the use of dyes, not as a tool for a grower, but as a tool for agricultural extension or consultation (really, anyone that wants to research or teach the safe and effective use of crop inputs)? Several of the cons are minimized or even eliminated. Additionally, this new lens reveals several uses for dyes beyond spray coverage. This is not an exhaustive list:
- Off-target (primarily drift) evaluation
- Dermal exposure / PPE evaluation
- Rinsate / sprayer cleanout evaluation
- Sprayer loading / point source contamination evaluation
I decided to compare a few of these dyes. I enlisted the help of a local blueberry operation. Being October, all the berries have been picked so we could spray the bushes without any risk to the fruit. Plus the sprayer was clean and the growers were curious to evaluate their spray coverage.

Having secured a location, spray equipment, and operator, I needed dyes and some criteria for choosing them. First and foremost, I chose fluorescent dyes that glowed under UV (aka black lights). My thinking was that they would be more interesting in demos, and given that we might be spraying horticultural operations, I didn’t want obvious and persistent stains on the produce. At least not something easily seen in daylight before it broke down and/or was washed away.
My UV dye candidates had to be:
- Moderately inexpensive.
- Non-toxic (i.e. had an SDS that clearly permitted human exposure, were environmentally friendly and could be sprayed on edible crops).
- Readily available in Ontario (e.g. quickly and cheaply shipped from within Canada or perhaps the US).
- Available in formats that facilitated small volume batches (anywhere from 50 mL squirt bottles for indoor demos, up to 50 L volumes for field demos).
- Clearly visible on plant tissue.
I found five likely prospects for the study. I won’t list prices, but none of them were over $100.00 CAD. Number 3 was a free sample and number 5 was gifted to me by a colleague more than 15 years ago. I looked up the SDS for that last one and was surprised that it was relatively inert. So, I used it.
| Dye number | Name of dye | Commercial size | Manufacturer | Location |
| 1 | IFWB-C81PT | 1 pint | Risk Reactor | California, USA |
| 2 | UVTRACER-G1PT | 1 pint | Risk Reactor | California, USA |
| 3 | Eco Pigment Blaze Orange SPL15JX | Sample size – 100 grams | DayGlo | Cleveland, Ohio, USA |
| 4 | Fluorescent Yellow Tempura Paint | 1 liter | Tri-Art Manufacturing | Kingston, Ontario, CA |
| 5 | Phosphor Powder (Zinc Orthosilicate: Manganese CAS#11-47-2) | 1 kg | Global Tungsten and Powders Corp. | Pennsylvania, USA |
I also purchased UV lights. When I was bequeathed the phosphor powder it came with heavy, ancient, black lights. They made an unsettling humming noise and required a power source, making them unwieldly for field work. I opted to try three battery powered versions instead. Again, I won’t list prices, but they weren’t unreasonable.
| UV flashlight number | Name of light | Manufacturer | Wavelength / wattage | Batteries |
| 1 | Super Tac | Risk Reactor | 395 NM / 850 μW/cm2 at 5 inches | Rechargeable battery provided |
| 2 | Mini Zoom | Risk Reactor | 395 NM / 1 watt | 1 AAA |
| 3 | V3 UV Flashlight with 68 LEDs | Amazon.ca | 395 NM / 10 watts | 3 AA |
Regarding the recipes, one of my criteria was that the dyes could be mixed in relatively small batches. I chose 50 L as the high end because the airblast sprayer we were using (Turbo-Mist 30P) could still prime when only 50 L was added to the tank. This allowed us to mix as small a batch as possible, while still having enough to spray a row of berries from both sides. We left three rows between treatments to serve as buffers.

I also had to consider the nature of the dyes. The Eco Pigment (Dye 3) is a hydrophobic powder and two colleagues warned me that it was notorious for plugging filters. So, it had to be mixed with a non-ionic surfactant (NIS) to help “wet” the powder prior to adding it to the tank. In fact, NIS seemed like a good idea for all my dye candidates, so I included Activate Plus (Sollio Agriculture, Winfield Solutions) in each recipe.

I added the dye, NIS, and a small amount of water to a Pyrex measuring cup on a digital scale, then rinsed the cup into a final volume of 50 L while filling the tank. I didn’t always follow the advice I received, so I’ll show you the ratios I was told and (right or wrong) what I ultimately did.
| Dye number | Manufacturer- or colleague-suggested ratio | Amount of dye | Amount of NIS | Amount of water |
| 1 | 1 part dye : 10,000 parts water | 125 mL | 65 mL | 310 mL |
| 2 | 1 part dye : 10,000 parts water | 125 mL | 65 mL | 310 mL |
| 3 | 1 gram dye : 1 mL NIS : 200 L water | 65 grams | 65 mL | 425 mL |
| 4 | 1 part paint : 100 parts water | 500 mL | 65 mL | 0 mL |
| 5 | 1 gram dye : 1.25 L water | 65 grams | 65 mL | 425 mL |
It took roughly 15 minutes to fill, prime, spray, and rinse out each dye. We started at 5:00 p.m., were done at 6:15, and then waited for sunset at 7:30.


We used a smartphone (Google Pixel 9a – 48 megapixel camera) to photograph each combination of dye and flashlight. It was tricky to find an angle where the black light illuminated the residue, but didn’t wash out the photo. In those cases where the dye was evident, it was always far more vibrant in person than through the lens of a camera. As for the results?
Lets start with the lights. We found that the high wattage of Light 3 showed dye more easily. This also happened to be the cheapest light, which was a pleasant surprise.
Dye 1 and 2 were disappointing. We couldn’t see anything on the plants. This dye is intended for monitoring plumbing and water courses, and the manufacturer states that the colour will disappear if the solution is mixed with chlorine. Perhaps mixing it with city water caused it to fade, but that’s likely to happen, so these dyes failed.


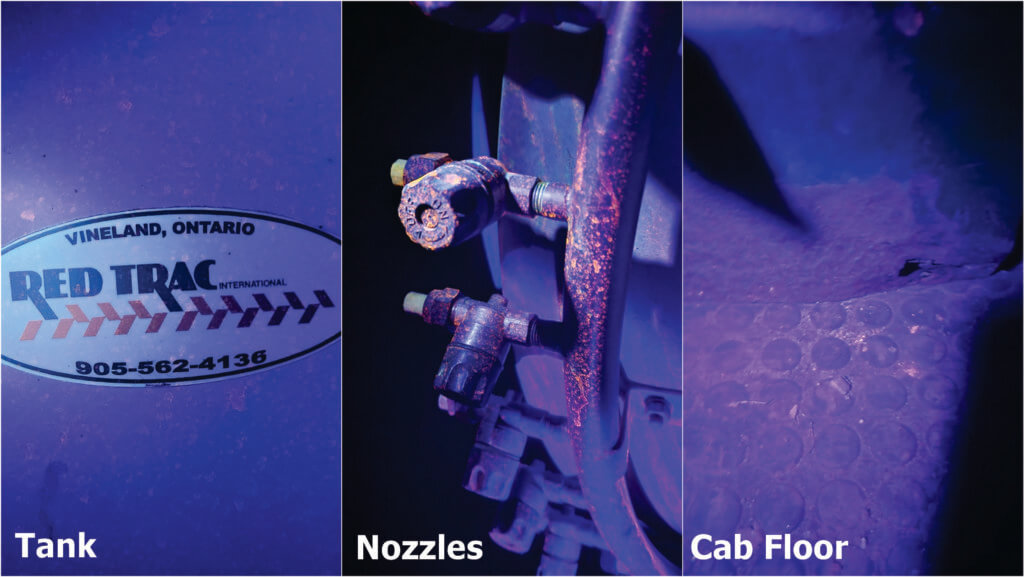
Dye 3 was spectacular. Not only was it evident with every light source (including day light to some extent), but we were able to find it several rows downwind, on the sprayer nozzles, all over the tires and on the floor of the cab (which surprised the operator). I may have mixed this one too strong; It seemed to clump on the leaves, but perhaps that’s because they were exceptionally waxy.



Dye 4 came in second place. It wasn’t amazing, but it was visible. This is children’s tempera paint, used in daycares for finger painting and at universities for raves. I’ve used it in the past with mixed results, not only to spray canopies, but in classroom demos on cabbage leaves and as a surrogate tracer to hunt down where pesticide hides in sprayer plumbing. It’s OK in a pinch if you mix it at least 2x more concentrated than I did here.


Dye 5, like dyes 1 and 2, was a disappointment. I’ve seen it used in powder-form to demonstrate how dermal exposure can spread as you touch clothing, doorknobs, your face, and places where the occasional adjustment is required. But in a liquid solution, it wasn’t any good at all.

Persistence
We followed up after the application to see if the dyes would persist. Twenty four hours after application, Dye 4 (our runner-up) was gone. This was no surprise given it was a water soluble paint and wasn’t terribly showy to begin with. However, Dye 3 (our winner) was still clearly in evidence. This is a hydrophobic, micro ground powder (~0.1 micron). That’s one reason it had to be mixed with a non-ionic surfactant. The following photos shows little or no change after 24 hours and a respectable dew:

Three days after application (DAA), we had a rain event. Four DAA this (blurry, sorry) image was taken:

We see that the deposits did redistribute to drip points and the overall coverage was reduced, but it was still holding on. This means it likely shouldn’t be used on any horticultural crop that isn’t going to be washed. Or at least used long before any fruit, leafy green or vegetable contacted by the powder will be harvested. Not because it is unsafe (see safety data sheet) but because of the optics to buyers.
Conclusion
And so, I hope you have been inspired by this process. I’ve learned that the use of dyes for education and research is potentially powerful, relatively cheap, and more accessible than I originally thought. Certainly the growers were impressed by what they could suddenly see and it’s led them to reassess some of their practices. Just bear in mind the possible persistence, and remember to wear gloves when mixing.

Thanks to Mark Ledebuhr, Helmut Spieser, David Manktelow, and Ben Werling for the helpful advice. Thanks to Brandon and Jordan Falcon for use of their spray equipment and their blueberry operation.
