It’s common advice: Test your water before using it as a spray carrier. You dutifully sample the well or dugout and await lab results. And what comes back is a whole lot of numbers. How to make sense of it all?
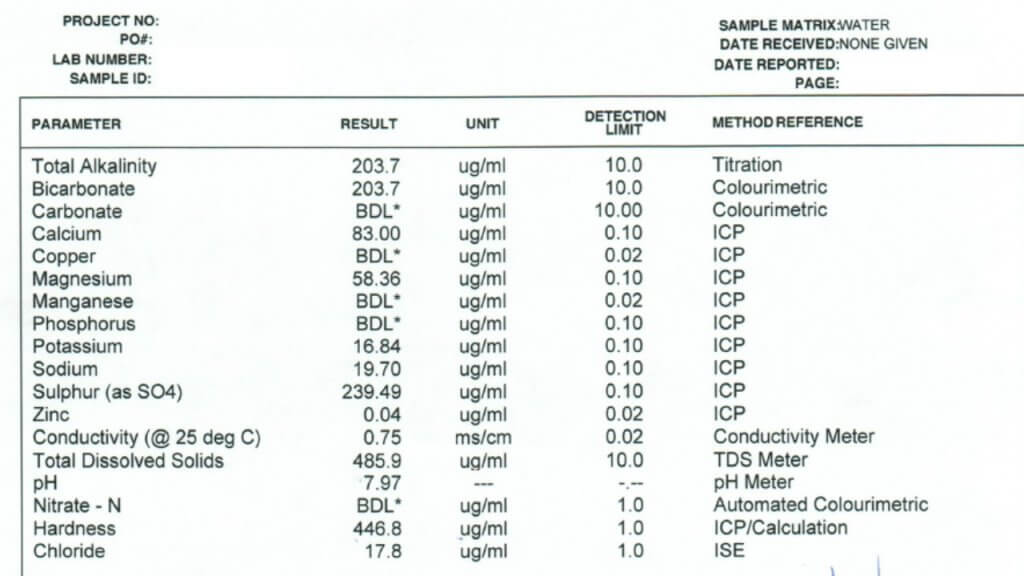
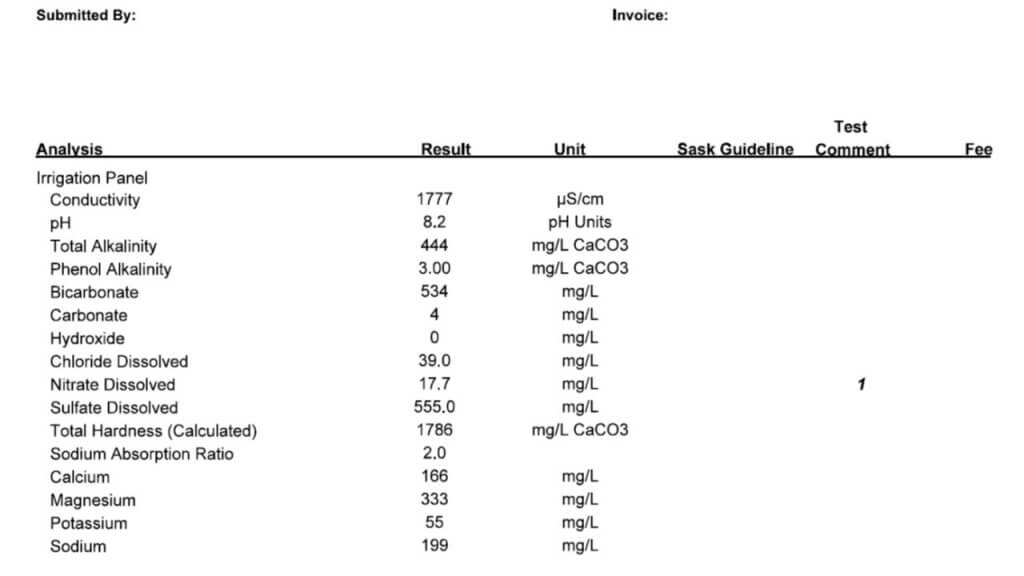
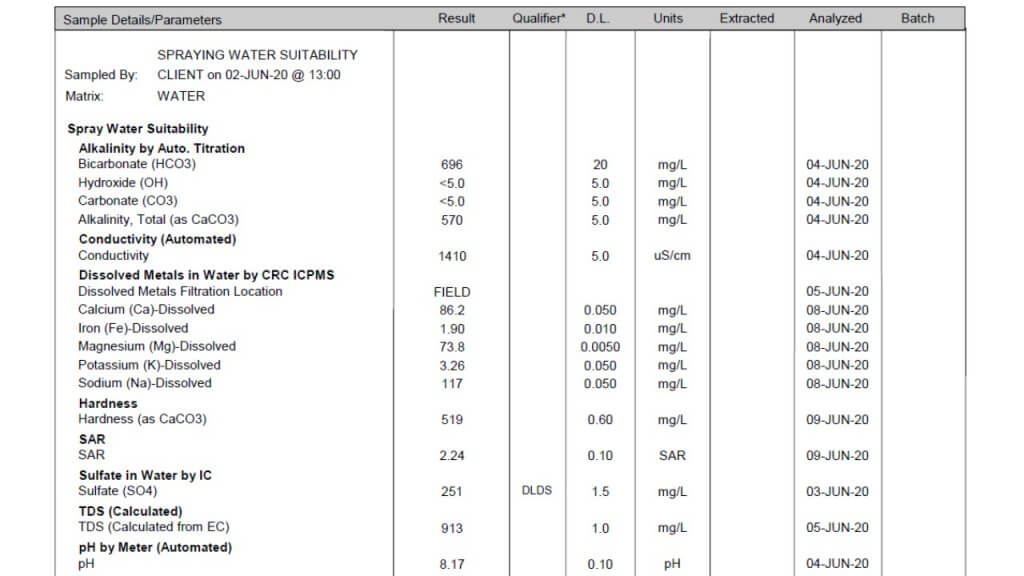
All three of these tests report a large number of properties and identify specific minerals and other solutes. Which ones are important in spraying? Here is the order in which I look at the numbers.
Conductivity: This property is usually expressed as micro Siemens per cm (µS/cm) and simply identifies how many ionic solutes are in a sample (watch for alternate units such as mS/cm and convert if necessary). It doesn’t differentiate between any minerals or other molecules, and therefore has limited information. But it does tell us if there is a large or small issue with water quality. If conductivity is below 500 µS/cm, the water is probably good for spraying. If the value is around 1000 to 2000, further investigation is necessary. Some water samples return conductivity of more than 10,000 µS/cm, and it’s important to identify which salts are causing that problem.
Note that Total Dissolved Solids (TDS) are often listed, and these are related to conductivity. A common way to get TDS is to multiply conductivity by 0.65. The conversion factor depends on which salts are dissolved but the bottom line is that TDS and conductivity are closely related.
Bicarbonate: Bicarbonates are HCO3 and their concentration is measured in milligrams per Litre (mg/L), which is the same as parts per million (ppm). Bicarbonates can antagonize Group 1 modes of action and the common threshold is 500 ppm. Research at NDSU has shown that Urea -Ammonium-Nitrate (UAN or 28-0-0 liquid fertilizer) can reduce bicarbonate antagonism in some Group 1 herbicides.
Total Hardness (calculated): This is one of the important parameters. Hardness antagonizes most weak acid herbicides, most importantly glyphosate and Liberty, and also ties up surfactants and emulsifiers which can result in problems with mixing and compatibility. Hardness is caused by five main cations, in order of strength these are iron (Fe++), magnesium (Mg++), calcium (Ca++), sodium (Na+), and potassium (K+). Of these, Mg and Ca are typically most abundant, although some water is high in Na.
The Total Hardness (ppm) reported in water tests is done by taking the most common two cations, calcium and magnesium, and using this formula: 2.497*Ca + 4.118*Mg. Note that some tests report hardness in Grains per Gallon, in this case, multiply grains by 17.1 to get ppm.
While this calculation usually gives an accurate prediction of hardness, you may need to have a look at iron and sodium as well. Iron is less common, but some well waters are high in sodium and neither mineral is captured in the Total Hardness parameter. A water test low in Total Hardness may be high in sodium, these are typically the samples with high conductivity.
The threshold for Total Hardness depends on the herbicide, its rate, and the water volume. The most common quoted values are 350 ppm for the lower rates of glyphosate (1/2 L/acre equivalent), and 700 ppm for the higher rates. Lower water volumes increase the concentration of herbicide, and reduce the impact of water hardness or bicabonates.
pH: This parameter is a bit over-rated because it is later affected by the herbicide and adjuvant dissolved in it. There is usually no concern with pH between 6 and 8, and water is rarely outside this range. It is best not to change the pH of water unless it is required on the label for mixing, because some products require low, and others require high pH for optimum solubility. Compatibility is an ever greater concern as our tank mix complexity increases.
Water Conditioners
The most common water conditioner is ammonium sulphate [AMS, (NH4)2 SO4]. In its pure form (21-0-0-24), a concentration of 1% to 2% w/v (8 to 17 lbs AMS/100 US gallons of spray water) solves most hard water and bicarbonate issues. Be cautious of using too much AMS (>3%), when added at high concentrations to some herbicides it can burn crops.
Research has shown that AMS works in two ways: The sulphate ion binds with hard water cations, forming an insoluble precipitate that prevents the antagonistic cations from binding to, and inhibiting, the herbicide. The ammonium ion has been shown to improve cellular uptake by weak ion herbicides.
Some product labels call for UAN as an adjuvant. UAN contributes ammonium, but not sulphate ions. As a result, while it may improve herbicide performance, it does not remove antagonizing cations from the mixture.
Acids have been used to combat hard water. In an acidic spray mixture, the weak acid herbicides become protonated. This means the herbicide’s negative charge at neutral pH becomes a neutral charge at acidic pH. In theory, the absence of the negative charge makes it harder for the antagonizing cations to bind to the herbicide. However, acids that work in this way are less effective at ameliorating the effect of hard water than AMS.
A small group of acids that includes citric acid and sufphuric acid can sequester or bind with hard water cations. But they do not contribute the ammonium ion that assists in weak acid herbicide uptake.
If your water is questionable for spraying, there are four common choices:
- Select a different well or dugout
- If the problem is barbonates or hardness, treat water with a conditioner such as Ammonium Sulphate (AMS), available in pure form as 21-0-0-24. Some acids (citric, sulfuric) can form conjugate bases with hard water cations, removing them from solution. But the associated significant lowering of pH should be treated with an abundance of caution as it may affect solubility of some pesticides.
- Reduce water volumes or increase herbicide rates.
- Use a municipal treated water source or invest in a reverse-osmosis (RO) system. RO is neither cheap nor fast and requires additional investment in storage, and a way to deal with solute-enriched waste water. But it may be the best option for some.
An Ammonium Sulphate calculator, originally developed by Winfield United using data from NDSU, can be downloaded here:
An excellent resource for adjuvant and water quality topics is this addendum in the North Dakota State University Guide to Weed Control.
Using good quality water lowers the likelihood of problems with mixing and overall performance and that pays significant dividends later.