This article was co-developed by Mike Cowbrough, OMAFRA Weed Management Specialist in Field Crops
Why scrub filters?
Why do we ask you to manually scrub residue from sprayer filters and housings before changing chemistries? Here are three reasons why rinsing in-place may not be good enough:
- There is potential for biologically-active levels of residue to persist in filters, even after a triple rinse, that could harm the next crop sprayed.
- Persistent residues could cause physical antagonism with the chemistry you use next. This can cost time and/or efficacy should it plug filters and nozzles or reduce spray uniformity.
- Persistent residues could cause chemical antagonism with the chemistry you use next – even several batches later. This could harm crops when the residue carried over from a much earlier application suddenly becomes soluble again thanks to detergents or pH adjusters in subsequent tank mixes.
An experiment
To some, the previous statements may seem excessive. Many sprayer operators claim that scrubbing filters is time consuming, or that they’ve never had a problem before, or that the tiny amount of residue they see in the filters after rinsing couldn’t possibly cause damage. We decided to test the efficacy of rinsing filters without removing them.
We constructed a table-top system that could circulate chemistry through a 50 mesh filter. Think of it as a scaled-down sprayer that returns solution to the tank rather than spray it out. It replicates what the line filters on a larger sprayer might experience during a typical spray day.

The method
The tank (i.e. the bucket) would be filled with a tank mix and circulated through the filter to replicate a spray day. The contaminated filter could then be sampled to establish a baseline, and then alternately contaminated and rinsed in place to compare how much residue remained. Specifically, we would drop the filter housing and scrub all surfaces in 500 ml of water to collect any and all residue.
Each sample collected would be poured through a filter for a visual check of residue. A small volume would be reserved to be sprayed on soybean and white bean seedlings as a bio assay of activity.
The process
We used Sencor (metribuzin) mixed at a rate that represented the low end of the label: 250 grams of product per acre at 5 gallons per acre. Not knowing what to expect, we circulated the solution through the filter for 20 minutes pumped at a rate of 1 gallon per minute and peeked into the tank.

Seeing that we were creating foam, we decided to add defoamer. Then we peeked into the filter housing to see what had accumulated so far.

Finding very little in the way of residue, we chose to let the system circulate for an hour. We felt this would represent a single real-world tank’s worth of product. Since we’d added defoamer, we decided it was safe to leave the lab and let the system circulate…

Despite having added defoamer, we had a mess to clean up. When we opened the bucket we noted all the product clinging to the lid (see below). We collected some of this scum to replicate what might be clinging to parts of the spray tank that are not adequately covered by rinse-down nozzles. We then dropped the filter into 500 ml of water and scrubbed the housing and filter to collect any and all residue.

We then added additional defoamer and checked in regularly as we circulated the mixture for several hours to replicate a full day of spraying. This time when we checked to see how much residue we had collected, found a surprising amount.

We replaced the filter and performed a triple rinse with water before dropping the filter to collect our residue sample. As shown below, the triple rinse cleared much of the residue, but trace amounts were still visible.

The 5 Second Squeeze
We refilled the tank with Sencor and defoamer and circulated it for several hours to once again contaminate the filter. This time, however, we added detergent to the second rinse. We did this in response to claims that Dawn dish detergent removed residues from dry products such as Atrazine without having to drop the filters.
A former agrichemical rep explained that the practice likely originated in Western Canada some years ago when several growers suddenly experienced physical incompatibilities with a particular batch of dry product. It was suspected that the problem was due to abnormally cold temperatures during mixing, but the result was that many were left with solids in the tanks that could not be flushed. Agrichemical reps bought pallets of Dawn dish detergent from big box stores and found it broke the solids down sufficiently to flush the tanks. From there, it is likely growers started adding it during the rinse to facilitate cleanout. But, is the “Five second squeeze” a myth?
Results

We saw a visible reduction in the filmy residue left behind by Sencor in the filter housing and on the walls of the pop bottles used to store the rinsate. It was easy to see why the 5 second squeeze appeared to improve matters… but was there enough residue to still there to cause trouble?

We poured the rinsate from each sample through red cloth. There was little or no visible evidence of Sencor in the sample taken from the lid of the filter following an hour of spraying (left), or our baseline sample of a filter contaminated after an hour of circulation with no rinse (second from left). There was a great deal in the sample from the filter following “a day’s spraying” and a triple rinse (second from right), and less in the triple rinse containing detergent (right). These last two conditions are compared below.

A volume of the rinsate from each sample was reserved for bio assay on soybean seedlings. The filter in the spray booth was cleaned thoroughly between conditions.

The following images show that even when there was little or no visible residue, there was still sufficient activity remaining to injure, or in the case of the triple rinse with water, kill soybean seedlings.
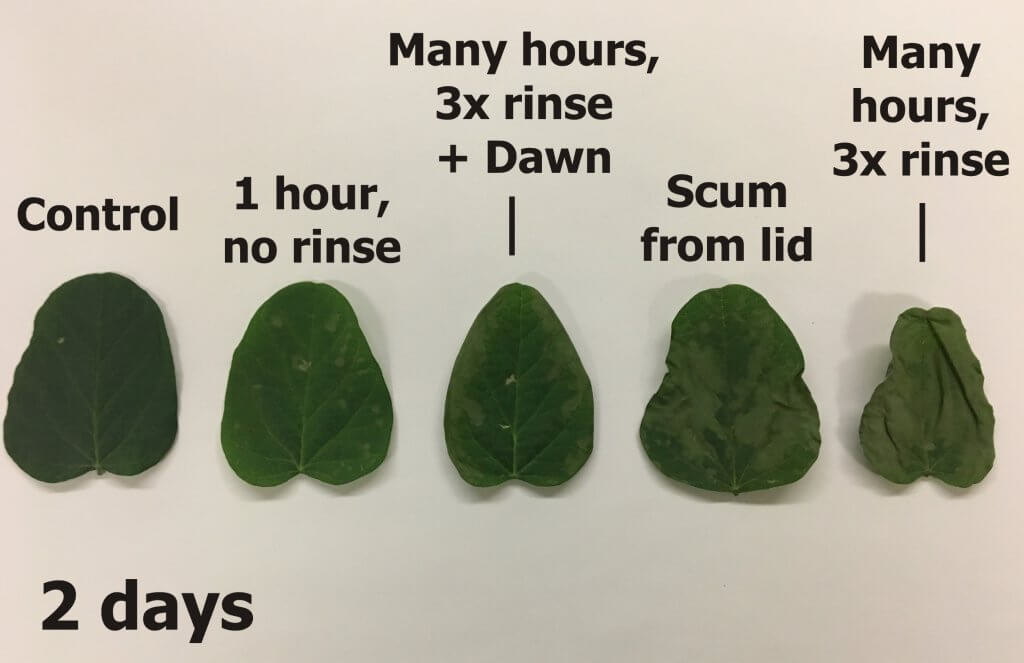


Summary
Bear in mind that this is a single experiment with a single chemistry, but it does support the following observations:
- Always rinse the sprayer as soon as possible and pay attention to dead-end plumbing and filters. Diligence is a function of knowing what was sprayed last, what is coming next, and the sensitivity of the crops being sprayed.
- Cleaners do not decontaminate – they loosen residues to make rinsing more effective. In our experiment, detergent appeared to reduce residue, but the bioassay showed sufficient activity remained to cause carry-over damage.
- A triple rinse with water may be insufficient to remove residue from filters. Even if the residue left behind does not cause damage in the next crop sprayed, it can persist and has the potential to react antagonistically with subsequent sprays.
Pro Tip
Not long after publishing this article, we were contacted by a grower who had difficulties with clay-based products plugging up his filters. It took a carry-over incident to convince him he needed to address the problem, so he installed $20 ball valves at the bottom of the filter housings. This isn’t as good as dropping and scrubbing filters, but opening and closing the valve under pressure during rinsing blew the filters clear of visible residue. Others have noted similar modifications on the pump of their tender truck to clear the filter of algae.
Other options include a hydraulic-style ball valve (stronger than plastic). Or, install a gator lock cam after the valve and insert a plug so if it’s accidentally opened it won’t dump the tank. Just keep a hose in the toolbox and insert it when you need to flush. Finally, one grower added Recently added a Thompson strainer to the sprayer and removed the screens from the Banjo Y’s. He ran a 1″ hose from the Thompson to a valve by the work station and cracks it open as part of every rinse.

