Download the 2023 publication from Crop Protection here.
Cercospora leaf spot (CLS), caused by the fungal pathogen Cercospora beticola, is one of the most damaging foliar diseases affecting sugarbeet (Figure 1) (Khan et al. 2008). Growers rely on broad-spectrum contact fungicides because they are less likely to cause fungicide resistance (OMAFRA 2020). However, these fungicides are usually less effective than other fungicides (Trueman & Burlakoti 2014), and require frequent reapplications (Thind & Hollomon 2018) and good coverage to be effective (Prokop & Veverka 2006; Roehrig et al. 2018).

We evaluated practices intended to improve the efficacy of Manzate® Pro-Stick™ (Mancozeb) by improving deposition and penetration into the sugarbeet canopy. Practices included different nozzle types (Shepard et al. 2006; Dorr et al. 2013), carrier volumes (Armstrong-Cho et al. 2008; Roehrig et al. 2018; Tedford et al. 2018) and the addition of InterLock®. InterLock is a spray adjuvant made with modified vegetable oil (MVO), vegetable oil and a polyoxyethylene sorbitan fatty acid ester emulsifier. It is intended to reduce the number of drift-prone, fine droplets without compromising the volume median diameter (WinField® 2019).
Research
In 2019 and 2020, InterLock and carrier volume were assessed to evaluate effects of:
- InterLock on Manzate Pro-Stick efficacy at different carrier volumes.
- InterLock on spray deposition and penetration within the sugarbeet canopy.
Objective 1: InterLock on Manzate Pro-Stick efficacy at different carrier volumes
Four replicated field trials were conducted at two sites, Dealtown (2019) and Ridgetown (2019 and 2020). Treatments were evaluated using four carrier volumes: 115, 235, 350, and 470 L ha-1 (12, 25, 37, and 50 gpa) and applied on a 14-day schedule.
Results
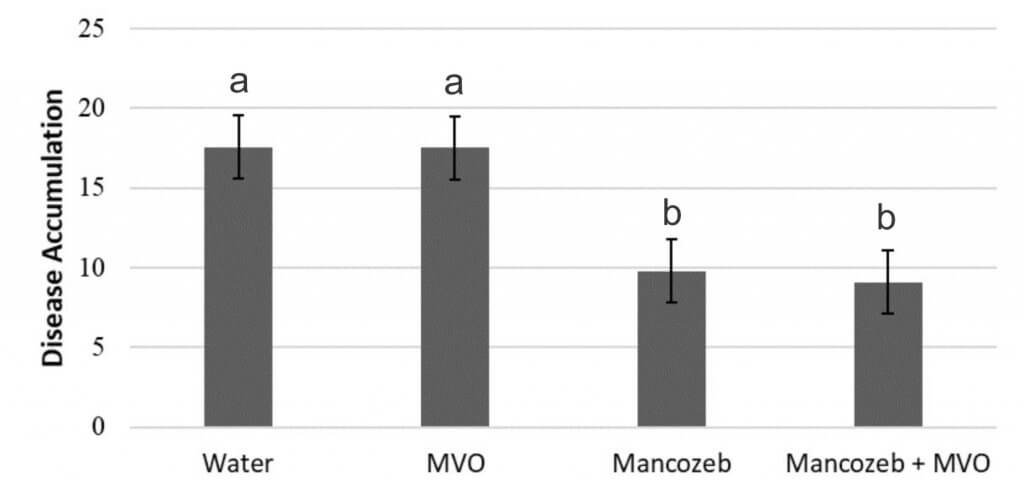
- Adding InterLock to Manzate Pro-Stick did not reduce disease accumulation over the season (Figure 2a) or improve beet and sugar yield or sugar quality compared to applications of Manzate Pro-Stick alone (data not shown).
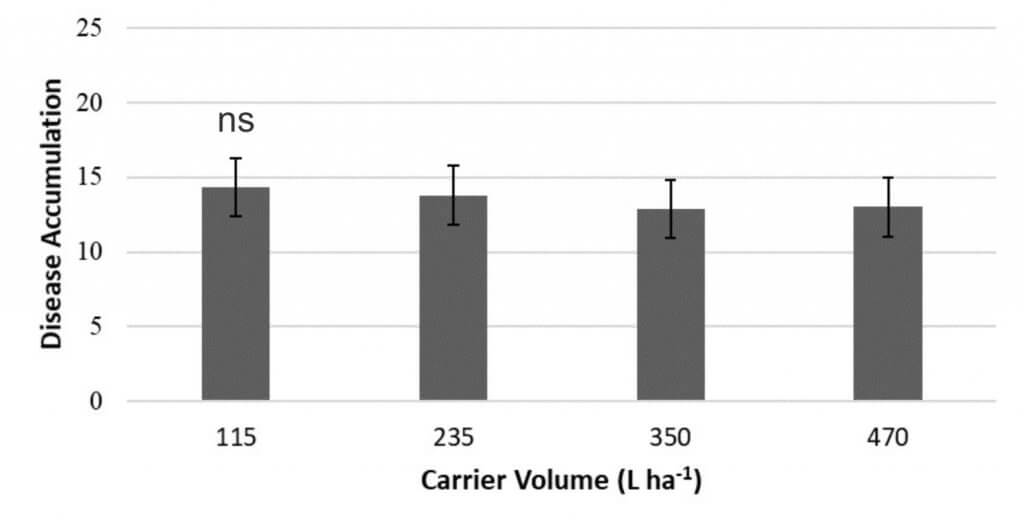
- Carrier volume did not affect disease accumulation (Figure 2b).
Objective 2: InterLock on spray deposition and penetration within the sugarbeet canopy
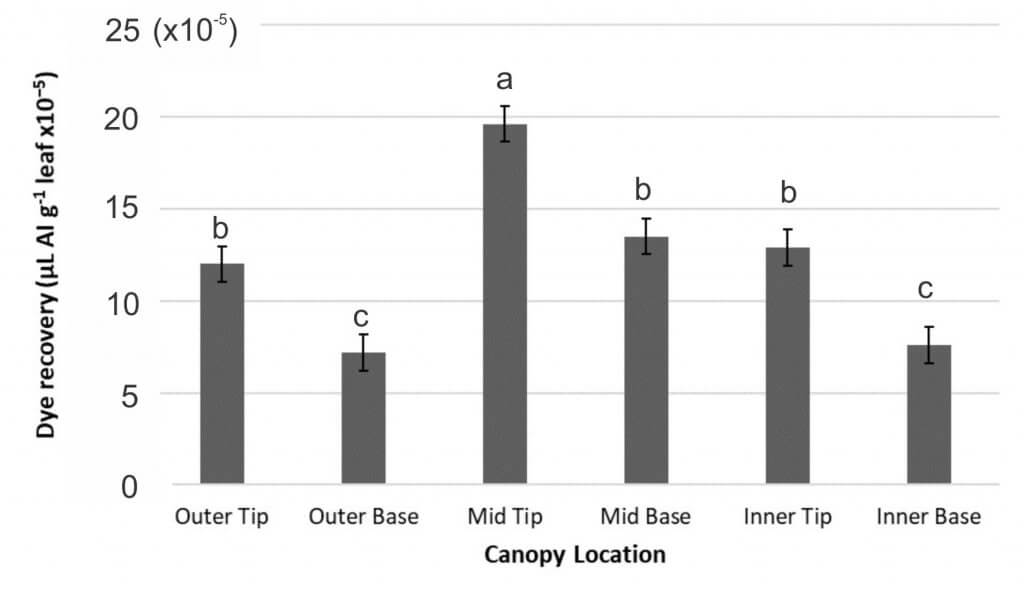
Deposition was evaluated using Rhodamine WT dye recovery. The amount of dye recovered for a treatment (µL AI/ g leaf tissue) was used to make assumptions about treatment deposition in the sugarbeet canopy. To assess spray deposition, samples were taken from six canopy locations (Figure 3 and 4).


Three sets of replicated experiments were conducted in Ridgetown (2019 and 2020) to evaluate the effect of InterLock on canopy deposition when 1) mixed with Manzate Pro-Stick, 2) using three different nozzle types, and 3) using three carrier volumes.
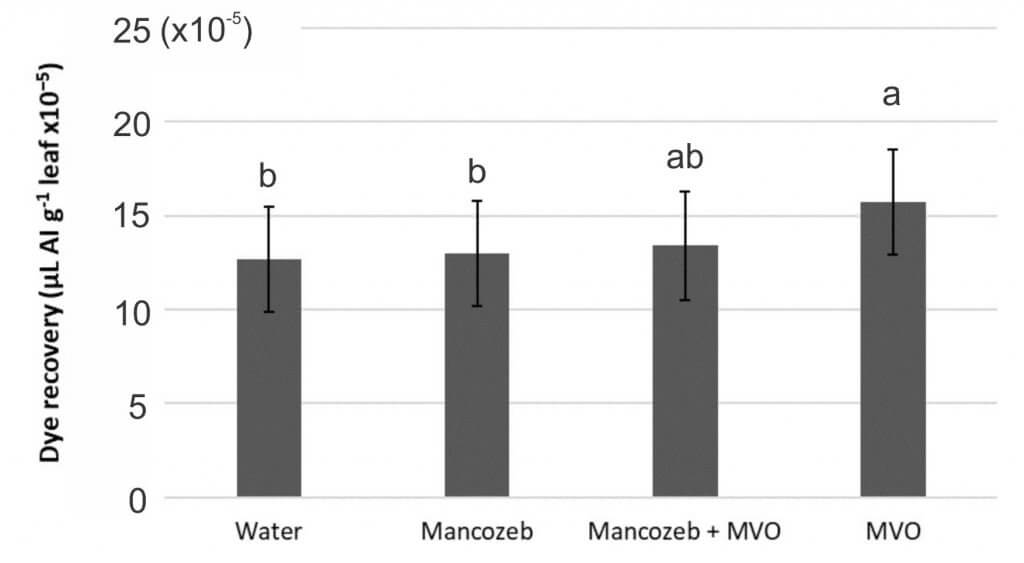
In the first study, four programs (Manzate Pro-Stick + InterLock, Manzate Pro-Stick alone, InterLock alone, and water) were evaluated for dye recovery.
Results
- Deposition was improved for the InterLock only treatment compared with water, but when InterLock was applied with Manzate Pro-Stick the deposition was the same as Manzate Pro-Stick applied alone (Figure 5). It is possible that the fungicide formulation or active ingredient had an antagonistic effect with InterLock, though we cannot determine that from this study.
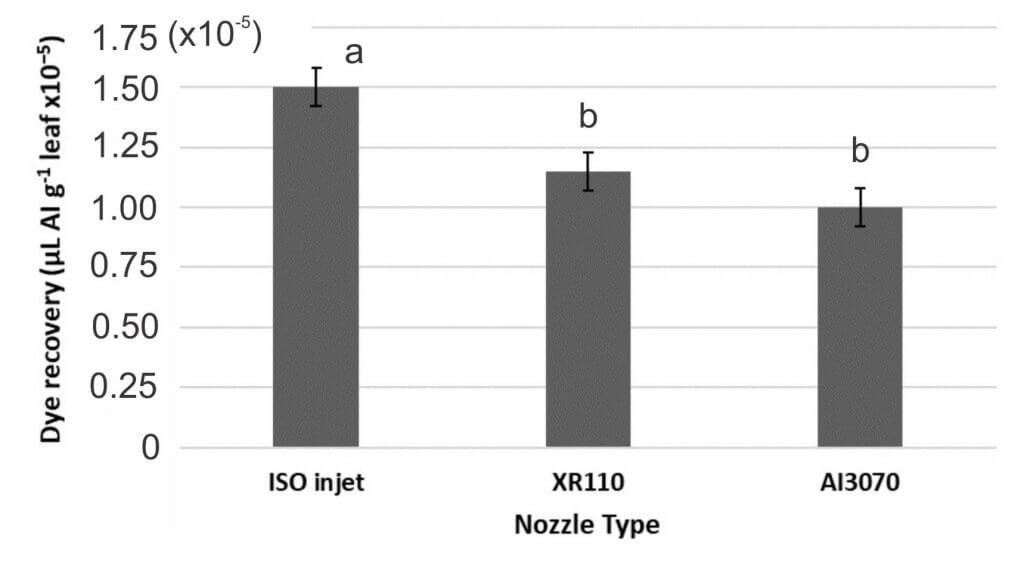
In the second study Manzate Pro-Stick + InterLock and Manzate Pro-Stick were applied using three different nozzle types at ~40 psi:
- The Hardi ISO Injet is an air inclusion 110° flat fan that produces a Very Coarse spray quality.
- The TeeJet XR110 is a conventional 110° flat fan that produces a Medium spray quality.
- The TeeJet AI3070 is an air inclusion, dual flat fan (30° and 70° spray angles) that produces a Coarse spray quality.
Results
- Adding InterLock did not affect deposition and did not alter the performance of any nozzle type (data not shown).
- Deposition among nozzles did differ, with the ISO injet nozzle providing improved deposition compared to the XR110 and AI3070 nozzles (Figure 6).
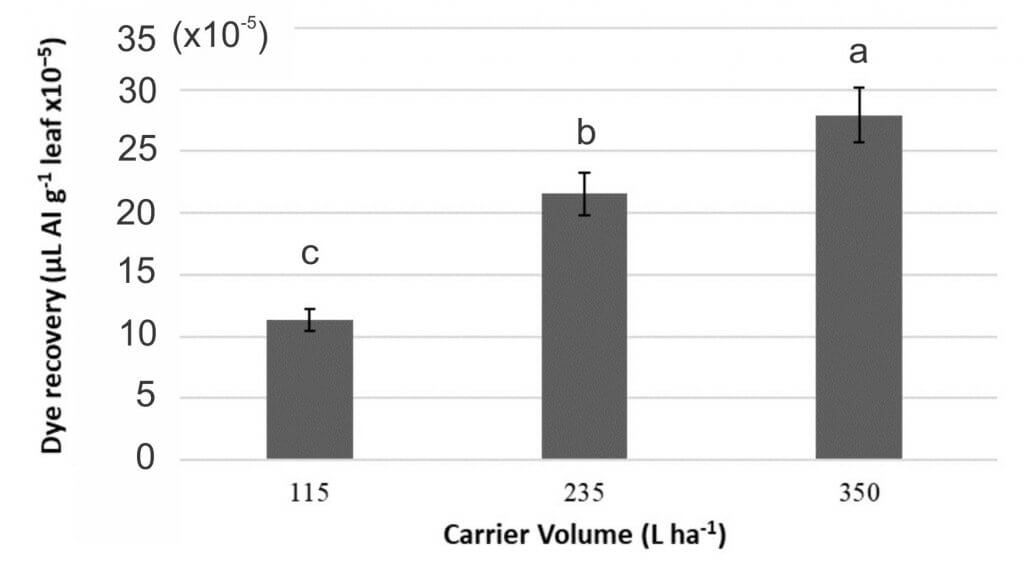
In the third study, Manzate Pro-Stick + InterLock and Manzate Pro-Stick were applied using three carrier volumes: 115, 235, and 350 L ha-1.
Results
- The addition of InterLock had no effect on deposition, regardless of carrier volume (data not shown).
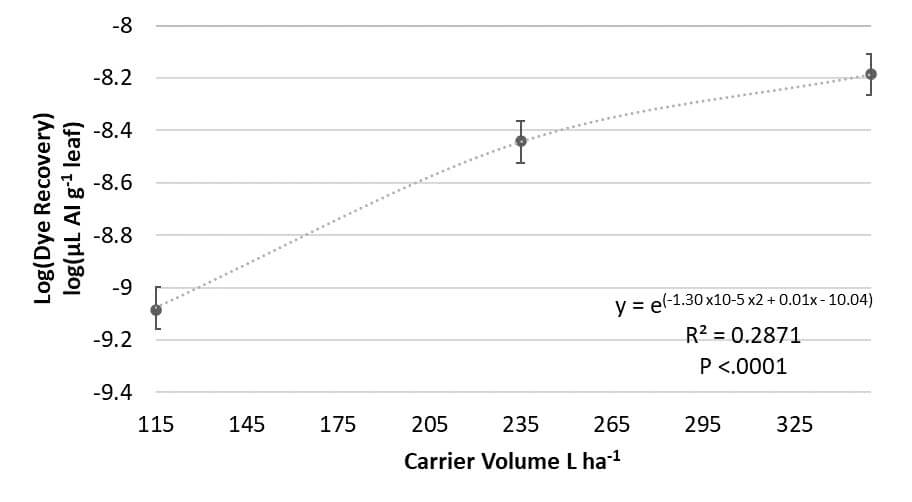
- Deposition increased with increasing carrier volume (Figure 7a). A regression analysis determined a curvilinear relationship between carrier volume and deposition, predicting that deposition would increase with increased carrier volume until a maximum carrier volume was reached (Figure 7b). Many studies indicate that at exceptionally high carrier volumes coverage can be reduced primarily due to run-off.
- Even though increased carrier volume improved fungicide deposition, increased volume did not improve fungicide efficacy for CLS management (Objective 1 efficacy trials).
Canopy location was an important factor in all experiments
The least deposition was always found in the outer and inner canopy from the base of the leaf, and in the outer canopy from the tip of the leaf (Figure 4), suggesting that these locations are the most challenging to achieve spray deposition. An example from the nozzle type experiment is shown in Figure 8. One of the proposed benefits of InterLock is for improved spray penetration, but in the current study, InterLock did not improve penetration of Manzate Pro-Stick into any of the harder to reach canopy locations.
Conclusion
Adding InterLock to Manzate Pro-Stick did not improve deposition in any field experiment regardless of the nozzle type or carrier volume used. Further, using InterLock with Manzate Pro-Stick did not improve fungicide efficacy for CLS management. However, we cannot determine from this study if InterLock would improve deposition, penetration, or fungicide efficacy using other fungicide products.
Despite findings of improved disease management with the use of larger carrier volume, fungicides are sometimes still applied with smaller carrier volumes of 100 L ha-1 or less (Armstrong-Cho et al. 2008; Roehrig et al. 2018) to save time and reduce the cost of application. In this experiment, increased carrier volume improved deposition but did not improve fungicide efficacy of Manzate Pro-Stick for CLS management. There is the potential that using increased carrier volume may be more beneficial in years with a greater disease severity, and may thus be worthwhile to growers, as has been observed in previous research on Cercospora leaf spot in Ontario (Tedford et al. 2018).
See the full thesis here.
Funding
This research was sponsored from the Canadian Agricultural Partnership, Ontario Agri-Food Innovation Alliance, Ontario Sugarbeet Growers’s Association, and the Michigan Sugar Company.
References
Armstrong-Cho C, Wolf T, Chongo G, Gan Y, Hogg T, Lafond G, Johnson E, and Banniza S. 2008. The effect of carrier volume on Ascochyta blight (Ascochyta rabiei) control in chickpea. Crop Prot. 27: 1020-1030.
Dorr GJ, Hewitt AJ, Adkins SW, Hanan J, Zhang H, and Noller B. 2013. A comparison of initial spray characteristics produced by agricultural nozzles. Crop Prot. 53: 109-117.
Khan J, del Rio LE, Nelson R, Rivera-Varas V, Secor GA, and Khan MFR. 2008. Survival, dispersal, and primary infection site for Cercospora beticola in sugar beet. Plant Dis. 92: 741-745.
Ontario Ministry of Agriculture, Food, and Rural Affairs (OMAFRA). 2020. Vegetable Crop Protection Guide, Pub 838. Sugarbeets. Queen’s Printer for Ontario, Toronto.
Prokop M, and Veverka K. 2006. Influence of droplet spectra on the efficiency of contact fungicides and mixtures of contact and systemic fungicides. Plant Protect. Sci. 42: 26-33.
Roehrig R, Boller W, Forcelini CA, and Chechi A. 2018. Use of surfactant with different volumes of fungicide application in soybean culture. Eng. Agr. Jaboticabal 38: 577-589.
Shepard D, Agnew M, Fidanza M, Kaminski J, and Dant L. 2006. Selecting nozzles for fungicide spray applications. Golf Course Manag. 74: 83-88.
Tedford SL, Burlakoti RR, Schaafsma AW, and Trueman CL. 2018. Optimizing management of Cercospora leaf spot (Cercospora beticola) of sugarbeet in the wake of fungicide resistance. Can. J. Plant Pathol. 41: 35-46.
Thind TS, and Hollomon DW. 2018. Thiocarbamate fungicides: Reliable tools in resistance management and future outlook. Pest Manag. Sci. 74: 1547-1551.
Trueman CL, and Burlakoti RR. 2014. Evaluation of products for management of Cercospora leaf spot in sugarbeet, 2014. Plant Disease Management Reports. 9: FC009.
WinField United. 2019. InterLock. [Internet]. [cited 2019 Feb 25].