This work was performed with Mike Cowbrough, OMAFRA Field Crop Weed Specialist.
In the early summer months, many field and specialty crop operations collect rainwater (or possibly pump water from holding ponds) into storage tanks for use as a carrier in spray applications. These tanks may be stationary, or they may be part of a nurse or tender truck that delivers both water and chemistry to the field as a means of improving operational efficiency.

In the case of translucent poly tanks, which are commonly used because of their light weight, custom shape, and low price point, light exposure will grow algae. Algal populations multiply exponentially and will clog spray filters and negatively affect filling. In response, growers use home-grown algicides such as copper sulfate, lengths of copper pipe, household bleach, chlorine, bromine, etc. They do so with little or no guidance and therefore little or no consistency. Beyond the obvious questions surrounding efficacy, it is unknown whether these adjuncts create physical or chemical incompatibilities in the tank mix. If so, there is the potential for reduced efficacy and/or crop damage.
We tested popular methods for algae control by inoculating a series of 10 L translucent plastic jugs with an algal population sourced from a southern Ontario holding pond. The population was left to acclimate and generally establish itself (aka colonize) before we introduced some form of control. Each jug was then gently stirred and emptied through a sieve for qualitative assessment.
In a parallel experiment, we introduced the same algicides to fill water and conducted spray trials. 10 L volumes were mixed with a field rate of glyphosate and sprayed on RR soybeans. Weed control was assessed and soybean yield measured for each treatment.
Algicide Efficacy Experiment
In each treatment, tap water was mixed with a micronutrient growth media (from the Canadian Phycological Culture Centre at the University of Waterloo). This was an unsterilized 10% WC(ed) solution intended to provide micronutrients for algal growth while minimizing fungal and bacterial growth.
The source algae were collected from the bottom of a holding pond from a farm in Guelph, Ontario. Algae were homogenized and equal parts added to each jug. The jugs were former 10 L pesticide containers thoroughly rinsed and sprayed with Five Star’s “Star San” non-rinse sterilizer. Tank solutions were gently bubbled (one bubble every 10-15 seconds) with air from an aquarium pump. Air was balanced using a manifold and introduced via diffusion stones at the bottom of each jug.

Treatments
Each treatment was tap water plus growth media inoculated with algae and exposed to a natural diurnal/nocturnal cycle unless otherwise indicated.
- Control (no algicide)
- Left in a shaded area (no direct sunlight)
- Household bleach (approximately 5.25% sodium hypochlorite)
- Container was spray-painted black to exclude light
- Ammonia
- “Scotch Bright” copper-coated scour pad. (copper is often introduced as copper sulfate at 1 cup / 1,000 US gal. or a short length of copper pipe)
- Bromine (sourced from a local pool supply store)
| Treatment Number | Treatment Name | Rate (/US Gal.) | Rate (% v/v) | Rate (/10 L final volume) |
| 1 | Control (no algicide) | |||
| 2 | Shaded | |||
| 3 | *Household bleach | 1/4 tsp | 0.00033 | 3.3 mL |
| 4 | Black container | |||
| 5 | *Ammonia solution | 1/4 tsp | 0.00033 | 3.3 mL |
| 6 | Copper-coated scour pad | |||
| 7 | Bromine | 1/32 ml | 0.000004 | 0.04 g |
Method
On July 12, jugs were loaded with water and growth media and inoculated with algae. They were bubbled gently for one week to establish a stable algal colony. On July 19, algicides were added, or transferred to shade or black-out conditions. On August 31 (approximately six weeks later), jug contents were gently stirred and filtered through white cloth for qualitative assessment.


Observations

(1) Control. Liquid poured slowly through cloth. Algae was still alive and healthy. It formed some clumps but was not as thick as other treatments.
(2) Shaded. Liquid poured fast and easily through cloth. Was particulate in texture rather than clumpy or gelatinous. Very little mass and entirely brown, suggesting it was dead.
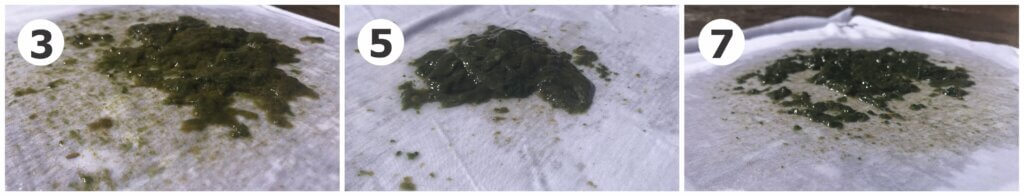
(3) Household bleach. Liquid poured easily through cloth until the clump of algae sitting at the bottom of the jug came out (i.e., most algae were not suspended). Thick mat of healthy-looking algae (note profile photo #3 below). Much greener and thicker than the control (1).
(4) Black container. Liquid poured fast and easily through cloth. Algae retained a little green coloration (more than the shaded condition (2)) but was particulate and not as healthy as the control (1). We intended for this treatment to exclude all light, but it was still able to enter at the bottom where the jug wasn’t completely painted. This may have kept the algae alive.

(5) Ammonia. Very difficult to pour liquid through the cloth (note profile photo #5 below). The only condition where a mat of algae was floating at the top of the jug rather than settled at the bottom. It was healthy, green and thick.
(6) Copper. The most gelatinous of all conditions, the liquid took the longest to pass through the cloth filter. While the algae seemed brown and dead, the gel would be very problematic during sprayer filling and spraying. Note that the copper scouring pad (shown unrinsed) has nothing growing on it.
(7) Bromine. Like the household bleach condition, liquid poured easily until the healthy mat of algae at the bottom of the jug came out (i.e., most algae were not suspended). Note profile photo #7 below.
Spray Efficacy Experiment
Ideally, adjuncts added to carrier water are inert. That means they don’t reduce a herbicide’s effectiveness on susceptible weeds or increase crop injury. For example, hypochlorite (found in bleach and in chlorinated water) reduces the biological effectiveness of low concentrations of isoxaflutole (the active ingredient in herbicides such as Converge and Corvus). However, when added to higher, agriculturally-relevant concentrations, the reduction in efficacy wasn’t considered significant (Lin et al., 2003). Conversely, bromide has been added to certain herbicides to improve performance (Jeschke, 2009).
There’s precious little information about synergistic or antagonistic effects from adding bleach, ammonia, copper or bromine to herbicide carrier water. To learn more, we added each of these adjuncts to the standard rate of glyphosate (900 gae/ha – 0.67 L/ac). Using a CO2-pressurized plot sprayer, the solution was applied to <10 cm tall weeds at 150 L/ha (15 g/ac) in glyphosate tolerant soybean at the 2nd trifoliate stage of growth (Elora Research Station, Ontario).
Visual crop injury was evaluated at 7 and 14 days after application. Weed efficacy was evaluated at 14 and 28 days after application. Soybeans yields were collected using a Wintersteiger plot combine and adjusted to a moisture content of 14%.
Weed Control
All treatments provided excellent control (>90%) of the weeds emerged at the time of application. Table 2 (below) presents the % visual control 28 days after application.
| Carrier Treatment (glyphosate 540 g/L at 900 gae/ha or 0.67 L/ac) | Lamb’s-quarter | Green pigweed | Witch grass | Green foxtail |
| 1) Control | 0 | 0 | 0 | 0 |
| 2) Shaded | 100 | 100 | 100 | 100 |
| 3) Household bleach | 100 | 100 | 100 | 100 |
| 3a) Household bleach – added prior to mixing | 95 | 97 | 100 | 100 |
| 4) Black container | 100 | 100 | 100 | 100 |
| 5) Ammonia | 100 | 100 | 100 | 100 |
| 6) Copper-coated scour pad | 100 | 100 | 100 | 100 |
| 7) Bromine | 100 | 100 | 100 | 100 |
Soybean Injury and Yield
There was no noticeable crop injury from any treatment (figure below) and yields were not significantly different from the control treatment (Table 3). However, when bleach was added prior to mixing, we did observe a trend in reduced soybean yield. We’re unable to explain this observation, but suggest it may be an unrelated issue (such as field variability). There were no obvious signs of crop injury, and the treatment provided excellent weed control.

| Carrier Treatment (glyphosate 540 g/L at 900 gae/ha or 0.67 L/ac) | Crop Injury (%)* | Avg. Yield (bu/ac) | Significance** |
| 4) Black container | 0 | 40.0 | A |
| 7) Bromine | 0 | 39.6 | A |
| 2) Shaded | 0 | 38.1 | AB |
| 3) Household bleach | 0 | 37.6 | AB |
| 1) Control | 0 | 37 | ABC |
| 5) Ammonia | 0 | 36.9 | ABC |
| 6) Copper-coated scour pad | 0 | 36.1 | BC |
| 3a) Household bleach – added prior to mixing | 0 | 34.0 | C |
Discussion
We elected to use an extreme situation where a single application of algicide was applied to an established, healthy colony. It’s possible that regular applications of algicide in a volume of water with little or no algae could maintain that condition.
A treatment was considered effective if it slowed or halted algal growth, especially if it also degraded algal populations, causing them to become brown, thin, and/or particulate. Once in the spray tank, the shear forces created by circulation should disperse any dead or degraded algal masses, making it easier to pass them through filters and nozzles.
The shade treatment appeared to kill algae as well as cause degradation. Second place went to the black-out treatment, where some light was unfortunately allowed in. This would have continued to fuel photosynthesis in the unpainted portion at the bottom of the jug. Conversely, the black exterior likely raised temperatures above >20 °C, which depresses most algal growth and may have contributed to the degradation.
Copper appeared to kill the algae but also created a gel that would pose problems to filters. Unlikely to be bacterial, as copper is known to suppress bacterial growth, it could have been caused by diatoms; certain invasive species are known to form brown jelly-like material endearingly referred to as “brown snot” or “rock snot”. Alternately, and according to work by J. Rodrigues and R. Lagoa, alginate polysaccharide can form viscous aqueous dispersions (such as gels) in the presence of divalent cations (such as copper).
No treatment appeared to reduce herbicide efficacy or affect crop health. However, unexpectedly, the household bleach added prior to mixing may have reduced soybean yield. Given the limited number of replications and the single plot location, we suspect this was a field effect, unrelated to the treatment.
Take Home
Based on these results, a combination of shade and light-excluding materials (e.g. black paint) would be the ideal approach to algae control. It’s cheap, effective, and doesn’t require periodic management. Buying black tanks is a good choice, or you can paint them. What you should paint them with is a matter of debate and there’s a very good Twitter thread on the subject if you’re interested.
Algae in Ponds and Dugouts
We didn’t test this, but the question has come up and the best we can do is share some long-standing farmer wisdom. Some have used Aquashade dye to absorb the photosynthetic wavelengths and reduce algae buildup. Reputedly it is moderately successful. Another option is adding aluminum sulfate to the pond, and with a lot of agitation it should clarify in about 48 hours. Still others have added a few square barley straw bales to the water and found it to work surprisingly well (possibly an allelopathic response). Tie a rope to them and float them in the pond.
Citations
Jeschke, Peter. 2009. The unique role of halogen substituents in the design of modern agrochemicals. Pest Manag Sci, 2010; 66: 10–27
Lin, C.H., Lerch, R.N., Garrett, H.E. and M.F. George. 2003. Degradation of Isoxaflutole (Balance) Herbicide by Hypochlorite in Tap Water. J. Agric. Food Chem. 2003, 51, 8011-8014