This article summarizes a 2022 paper that can be downloaded here.
Grasshoppers are an integral part of rangeland ecosystems, but population outbreaks can cause significant damage to adjacent crops and forage. For example, cattle consume about 1.5-2.5% of their body weight in forage per day, so pound for pound, a grasshopper will eat 12-20 times as much plant material as a steer. This represents serious economic damage to the cattle industry, especially during times of drought when forage is already scarce.

Insecticides are the primary means for suppressing grasshopper populations, and they are typically applied on rangelands using conventional fixed-wing aircraft, per the United States Department of Agriculture (USDA) Animal and Plant Health Inspection Service (APHIS) Rangeland Grasshopper and Mormon Cricket Suppression Program. This is particularly effective when population hotspots can be targeted before those populations grow to critical numbers, but depending on the location, this short turn-around time can be challenging.
Recently, the use of remote piloted aerial systems (RPAS or drones) for spraying in small farm operations and for site-specific management of crop pests in terrains not easily accessible to fixed-wing aircraft has received increased attention around the globe. Drones have the potential to occupy this niche because they can fly (or even hover) closer to plant canopies with more precision and safety than conventional aerial systems.
Given these strengths, and given that swath uniformity is less of a critical issue when pests (such as grasshoppers) are particularly mobile, we investigated the efficacy of treating grasshopper population hotspots using a drone.
Plots
A randomized plot design with two treatments (untreated and control) and eight plots was established on rangeland near Estancia, New Mexico, with each rectangular plot measuring approximately 4 ha (10 ac).

The RPAS was a six-rotor Precision Vision 35 (Leading Edge Aerial Technologies, New Smyrna Beach, FL, USA) equipped with four Turbo TeeJet XR110-01 nozzles (two on each side of the aircraft) mounted to spray booms.

Swathing
Twenty-six water sensitive papers were placed in a line perpendicular to the flight path, approximately 0.91 m (3 ft) apart, to capture the spray deposition. The RPAAS was flown at a speed of 8.94 m/s (20 mph).
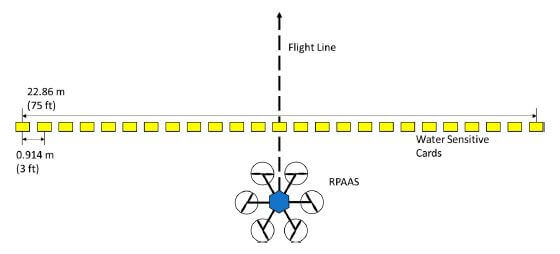
The papers were scanned with an Epson Perfection 1240U laser bed scanner and swath analysis was performed using DropletScan (WRK of Arkansas, Lonoke, AR, USA; WRK of Oklahoma, Stillwater, OK, USA; and Devore Systems, Inc. Manhattan, KS, USA). Papers were scanned at 600 dpi and the software was used to determine the coefficient of variation (CV) for both a simulated racetrack and a back-and-forth spray pattern.
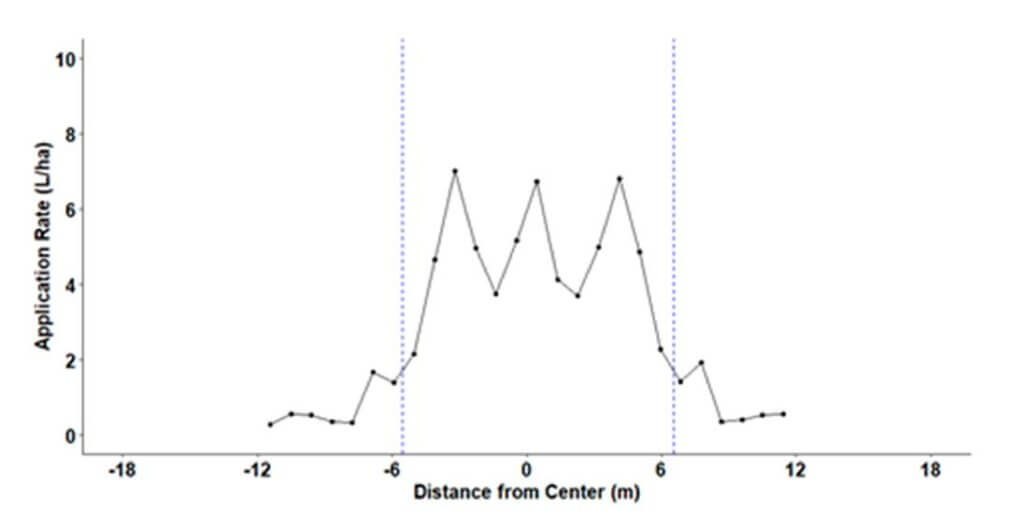
The CV for multiple effective swaths was calculated and the swath width with the lowest CV was chosen for the study. This effective swath width was 12.2 m (40 ft) with an average application rate of 2.75 L/ha (37.6 fl. oz./acre). Following a 50% RAAT IPM strategy, a 24.4 m (80 ft) swath was used for treatments, with an average treated swath application rate of 1.4 L/ha (18.8 fl. oz./acre).
Treatment and Efficacy
Two types of grasshopper population density estimation methods (visual estimation and sweep netting) were performed on the day before treatment (pre-count) and 3, 7, 10, and 14 days after treatment.
The RPAS was flown at 9.8 m/s (22 mph) at an altitude of 3.05 m (10 ft). Treatments with the liquid insecticide Sevin XLR PLUS required a single flight to cover each replicated plot, without completely depleting the battery, and each of the four treatments was completed within approximately five minutes of the total flight time.
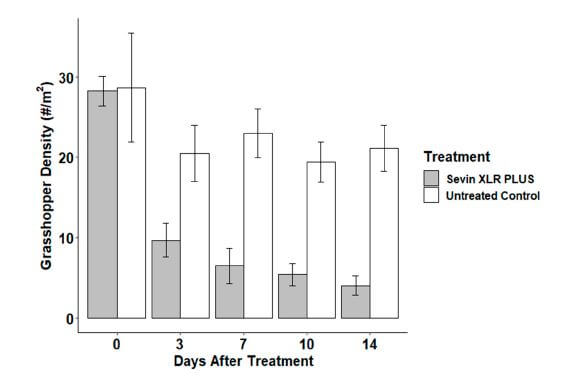
Our results demonstrated that Sevin XLR PLUS significantly suppressed grasshopper populations over a 14-day period (normalized population reduction was 79.1 ± 8.4% SEM) and quite rapidly (mostly by day 3) compared to untreated controls. These results are comparable to those achieved with fixed-wing aircraft.
Observations
Because RPAASs are relatively portable, the potential exists to shorten the average length of time between the identification of a hotspot and a treatment. The RPAS covered the whole test area in a single flight in approximately 5 minutes, making these population hotspot treatment applications relatively rapid, potentially more cost-effective, and more targeted in comparison to fixed-wing aircraft.
We would describe the observed efficacy as successful in the sense that grasshopper populations, when accounting for reduction in untreated control plots, were reduced by 79.1 ± 8.35% SEM. This is very close to what is typically expected for APHIS program treatments, which is 80 to 95% population reduction. Our lower-end results can probably be attributed to the arid conditions, lower levels of rangeland forage observed during the study in that region of New Mexico, and a mobile, rapidly aging population.
Before adoption as an application method option, further research is recommended on using an RPAS to cover larger areas in combination with using diflubenzuron-based insecticides, which are often preferred.
This research was funded by an interagency agreement with USDA-APHIS-Plant Protection
and Quarantine (PPQ): 20-8130-0893. It may not necessarily express APHIS’s views.